Ammonium Acetate 0.1% w/v Molarity - HPLC Primer
Date: 14-APRIL-2020 Last Updated: 1-DECEMBER-2025
Percentage given as w/v means weight over volume. This is typically used for solids dissolved in a liquid.
Ammonium acetate is a solid and is often used in HPLC mobile phases. Suppose you have a 0.1 % (w/v) ammonium acetate solution but your SOP requires you to state the concentration in mM. How do you convert? You will need the molecular weight of the solute (77.1 g/mol in this ca se.)
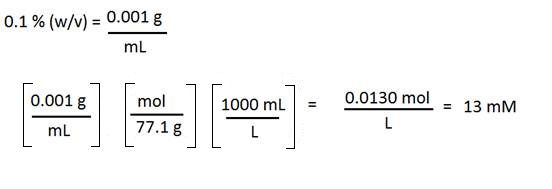
Percentage given as w/v means weight over volume. This is typically used for solids dissolved in a liquid.
Ammonium acetate is a solid and is often used in HPLC mobile phases. Suppose you have a 0.1 % (w/v) ammonium acetate solution but your SOP requires you to state the concentration in mM. How do you convert? You will need the molecular weight of the solute (77.1 g/mol in this ca se.)
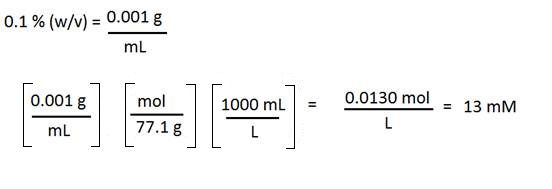
1. Convert the percent to grams per mL
2. Change grams to mol (use the molecular weight)
3. Change mL to L
4. You will get mol per liter, or M. This may be expressed as mM by multiplying by 1000.
2. Change grams to mol (use the molecular weight)
3. Change mL to L
4. You will get mol per liter, or M. This may be expressed as mM by multiplying by 1000.
