Steel Information for Low-alloy AISI Steels - TECH Information
Date: 14-FEBRUARY-2023 Last Updated: 26-OCTOBER-2025
The major code used to classify the various steels in the United States was developed by the American Iron and Steel Institute (AISI)
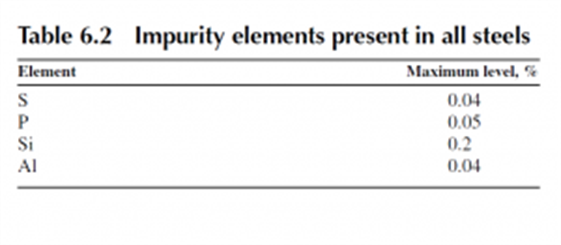
Manganese is present in all steels.
The amount of manganese present in a steel can only be found in a table of compositions, because the AISI code does not contain this information.
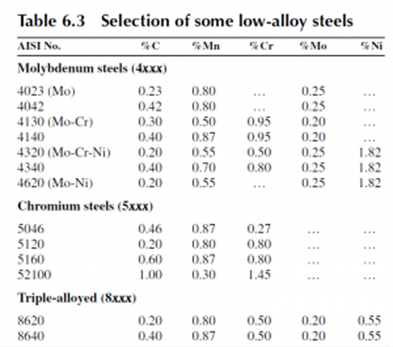
The common alloy steels have one or more of the three elements chromium, molybdenum, or nickel added, and these steels are referred to as the low-alloy AISI steels.
Manganese is present in all steels to overcome problems with sulfur embrittlement.
Sulfur is an impurity atom in steels that cannot be economically removed. It forms a compound with iron, iron sulfide (FeS), that is molten at the hot rolling temperatures of steel. The molten FeS wets the austenite grain boundaries and leads to brittle grain boundary fracture during hot deformation, a phenomenon known as hot shortness. The addition of manganese replaces the FeS compound with a manganese sulfide compound (MnS) that is not molten at the hot rolling temperature and cheaply overcomes the hot shortness problem.
The addition of manganese and the chromium, molybdenum, and nickel alloying elements change the position of the A3, A1, and Acm transformation lines from their locations on the binary iron-carbon phase diagram.
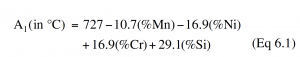
Equation 6.1 shows how the A1 line is lowered by manganese, raised by chromium and nickel, and not affected by molybdenum at its levels in the 4xxx steels. All of these additions reduce the composition of the pearlite point below 0.77%.
In general, these shifts are small and often can be ignored in designing heat treatments. However, as alloying content increases, they can be significant, as is illustrated for the heat treatment of 52100 steel.

The major code used to classify the various steels in the United States was developed by the American Iron and Steel Institute (AISI)
Manganese is present in all steels.
The amount of manganese present in a steel can only be found in a table of compositions, because the AISI code does not contain this information.
The common alloy steels have one or more of the three elements chromium, molybdenum, or nickel added, and these steels are referred to as the low-alloy AISI steels.
Manganese is present in all steels to overcome problems with sulfur embrittlement.
Sulfur is an impurity atom in steels that cannot be economically removed. It forms a compound with iron, iron sulfide (FeS), that is molten at the hot rolling temperatures of steel. The molten FeS wets the austenite grain boundaries and leads to brittle grain boundary fracture during hot deformation, a phenomenon known as hot shortness. The addition of manganese replaces the FeS compound with a manganese sulfide compound (MnS) that is not molten at the hot rolling temperature and cheaply overcomes the hot shortness problem.
The addition of manganese and the chromium, molybdenum, and nickel alloying elements change the position of the A3, A1, and Acm transformation lines from their locations on the binary iron-carbon phase diagram.
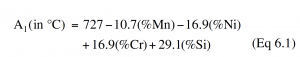
Equation 6.1 shows how the A1 line is lowered by manganese, raised by chromium and nickel, and not affected by molybdenum at its levels in the 4xxx steels. All of these additions reduce the composition of the pearlite point below 0.77%.
In general, these shifts are small and often can be ignored in designing heat treatments. However, as alloying content increases, they can be significant, as is illustrated for the heat treatment of 52100 steel.



