Fast and Reproducible Method
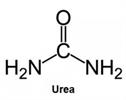
Urea is notoriously difficult to retain using conventional HPLC techniques. Due to its highly polar nature, it typically exhibits minimal or no retention on standard reversed‑phase columns.
This approach offers the advantage of being extremely simple, eliminating the need for time‑consuming derivatization or reaction steps required by alternative methods.
Peak:
Urea
Column: Cogent Bidentate C18™, 4μm, 100Å
Catalog No.: 40018-05P
Dimensions: 4.6 x 50mm
Mobile Phase: 15% DI Water / 85% Acetonitrile / 0.1% (v/v) Formic Acid
Flow rate: 0.2 mL /minute
Detection: UV @ 205 nm
Injection vol.: 5 μL
Sample Preparation: 3mg of Urea standard in 50:50 ACN:DI
Note: There is growing demand for a sensitive and reliable procedure for the determination of Urea in many matrices such as milk, soil extracts, seawater and wine. In addition there are several clinical applications for the analysis of this compound. The most common approach for measurement of Urea involves detection of ammonia (after hydrolysis) by color forming reactions – enzymatic, colorimetric Methods. The other techniques require noxious reagents and produce an unpleasant odor [1]. Newer Methods involve high-performance thin layer chromatography-densitometry, alkalimetric titration. HPLC is the most specific method but either organic Normal Phase Chromatography or Ion Pairing Chromatography have to be used to retain this very polar compound until this Method.
[1] “Determination of urea using HPLC with fluorescence detection after automated derivatization with xanthydrol”, S. Clark, P.S. Francis, X.A. Conlan, N.W. Barnett, J. Chromatography A, 1161 (2007) 207-213.
